Polyepoxides (Epoxies)
Properties
Epoxy resins, sometimes called polyepoxides, are a class of reactive prepolymers and polymers which contain at least one epoxide group. The resins can be reacted (crosslinked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids and acid anhydrides, phenols, alcohols and thiols. The co-reactant is often called hardener or curative, and the crosslinking reaction is commonly referred to as curing. The thermosetting polyepoxides are known to have good mechanical properties, and high temperature and chemical resistance. However, when cured with a curative, the mechanical properties are also dependent on the curative-polyepoxide ratio. An excess of amine usually leads to inferior mechanical properties.
To achieve the optimal performance properties, the epoxies are typically cured with stoichiometric or near-stoichiometric quantities of curative. As with other classes of thermoset polymer materials, blending different grades of epoxy resin, as well as use of additives, plasticizers or fillers is common to achieve the desired processing and/or final properties, or to reduce cost. Blending, and use of additives and fillers is often referred to as formulating.
An important criterion for epoxy resins is the epoxide content. The epoxide content is commonly expressed as the equivalent weight, which is the weight of resin containing 1 mole equivalent of epoxide (g/mol). The equivalent weight is used to calculate the amount of co-reactant (hardener) to use when curing epoxy resins.To achieve the desired performance results from an epoxy / amine curing agent system, it is important to use the resin and curing agent in stoichiometric amounts. To calculate the proper mix ratio, the following information is needed:
AHEW = Amine Hydrogen Equivalent Weight
EEW = Epoxide Equivalent Weight
The stoichiometric mix ratio or the parts by weight of curing agent per 100 parts of epoxy resin (phr) is given by
Phr = AHEW / EEW x 100
Epoxy Compounds
The most common and important epoxy compound is the diglycidyl ether of bisphenol A, which is formed from reacting epichlorhydrin with bisphenol A. The industrial grades normally contain some distribution of molecular weight, since pure DGEBA shows a strong tendency to form a crystalline solid upon storage at ambient temperature. If exactly two moles of epichlorhydrin are reacted with one mole of bisphenol A the product is the bisphenol A diglycidyl ether, commonly abbreviated to DGEBA. Increasing the ratio of bisphenol A to epichlorhydrin during synthesis produces higher molecular weight linear polyethers with glycidyl end groups, which are semi-solid to hard crystalline materials at room temperature depending on the molecular weight achieved. As the molecular weight of the resin increases, the epoxide content reduces and the material behaves more and more like a thermoplastic. Very high molecular weight polycondensates of 30 000 – 70 000 g/mol are known as phenoxy resins and contain virtually no epoxide groups since the amount of terminal epoxy groups is insignificant compared to the total weight of the molecule. These resins do however contain hydroxyl groups throughout the backbone, which may also undergo crosslinking reactions, i.e. they can react with isocyanates etc.
Curatives
The most important class of curatives are primary and secondary amines. They undergo an addition reaction with the epoxide groups to form a hydroxyl groups and a secondary / tertiary amines. The reactivity of primary amines is approximately double that of secondary amines. Typical curatives are alipahtic and aromatic (poly)amines, like diethylenetriamine (DETA), triethylenetetramine (TETA), tetraethylenepentamine (TEPA) to name only a few. The reactivity decreases in the order aliphatic amines > cycloaliphatic amines > aromatic amines. The temperature resistance generally increases in the same order, because aromatic amines form much more rigid structures than aliphatic amines, which are less susceptible to oxidation. The epoxies cured with aromatic amines have excellent properties. However, they are often more harmful (in the uncured state) than the aliphatic amines. For this reason, aliphatic or cycloaliphatic are often prefered as curatives. Other important amine curatives are polyamine adducts and polyamides, which usually have a (much) lower vapor pressure than the low MW amine curatives (less odor). However, the adducts and amides cure slower than the low MW (primary) amine curatives.
Another important class of curatives are anhydrides. These curatives cure much slower than amines and amides. For this reason, the amine-epoxy mixture is usually cured at elevated temperatures. The reaction occurs after ring opening of the anhydride by secondary hydroxyl groups in the epoxy resin. These epoxies have often excellent electrical properties and are used for high voltage insulators.
A third class of curatives are thiols (mercaptanes) which rapidly react with the epoxy. Thiols have usually a pungent odor and, therefore, are employed on a much smaller scale than the amine curatives.
Epoxy products cured with low MW curatives tend to have a high crosslink density which results in good resistance to heat and chemicals, including solvents. However, they are often brittle and possess poor flexibility and impact resistance. For this reason, the low MW curatives are often blended with other curatives. For more demanding applications, (reactive) rubber tougheners (ATBN, CTBN) are added to the mixture.
COMMERCIAL Epoxies
Two major manufacturers of epoxy resins are Huntsman and Hexion who sell these resins under the trade names Araldite and EPON. The two most widely used epoxy resins are diglycidyl ethers of bisphenol A (DGEBA) and diglycidyl ether of bisphenol F (DGEBF). These resins are sold as low MW liquids, solid resins, high MW (reactive) polymers dissolved in solvent and as prepregs. Commercial cured epoxy products are also available which are typically sold as glass fiber or mineral fiber reinforced sheets.
| Bisphenol Epoxy | Structure of Repeat Unit | Trade Name |
| Bisphenol A epoxy resin, Bisphenol A diglycidyl ether (DGEBA) |
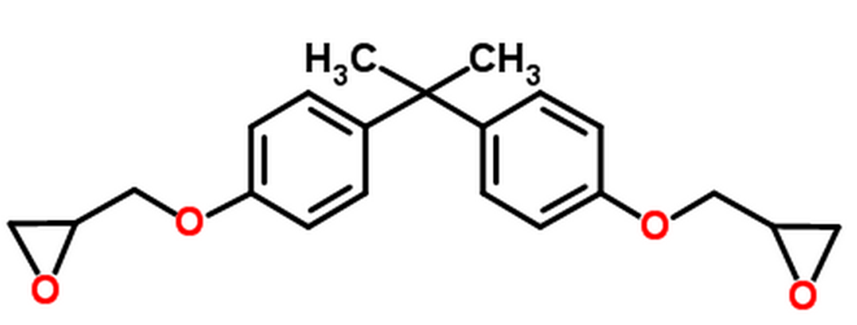
| Araldite, Conapoxy, D.E.R., Epolite, EPON, Epoxylite |
| , Bis(4-glycidyloxyphenyl)methane, Bisphenol E diglycidyl ether (DGEBE) |
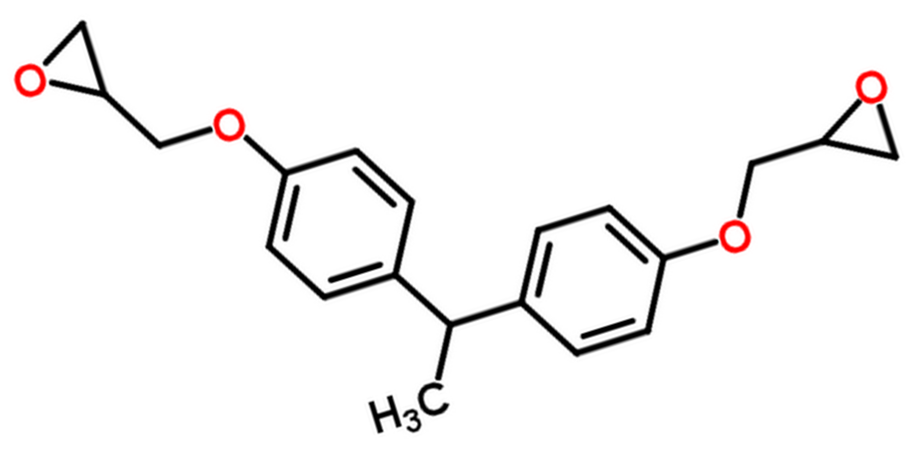
| |
| 2,2'-[1,1-Ethanediylbis(4,1-phenyleneoxymethylene)]dioxirane, Bisphenol F diglycidyl ether (DGEBF) |
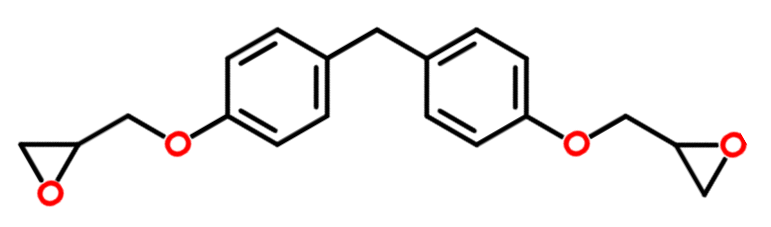
|
APPLICATIONS
Epoxies have a wide range of applications, including metal
coatings, potting compounds and encapsulation for electronics / electrical components, thermal interface materials (TIMs), and high voltage insulator materials.
They also find uses in fiber-reinforced plastics and
structural adhesives. Due to their excellent corrosion resistance
and bond performance, they are widely used in the automotive and
aerospace industry as crash-resistant adhesives.